Theme: Challenges And Opportunity In Pharmaceutical chemistry
Euro Pharma Chemistry 2019
Conference Series LLC Ltd welcome professional chemists, researchers, professors, scientific communities, delegates, students, business professionals and executives from all over the world to attend the 4th PHARMACEUTICAL CHEMISTRY Conference scheduled during June 27-28, 2019 in Amsterdam, Netherlands. Pharmaceutical chemistry is the study of drugs, and it involves drug development. This includes drug discovery, delivery, absorption, metabolism, and more. There are elements of biomedical analysis, pharmacology, pharmacokinetics, and pharmacodynamics. Pharmaceutical chemistry work is usually done in a lab setting.
Pharmaceutical chemistry involves cures and remedies for disease, analytical techniques, pharmacology, metabolism, quality assurance, and drug chemistry. Many pharmaceutical chemistry students will later work in a lab. Pharmaceutical chemistry leads to careers in drug development, biotechnology, pharmaceutical companies, research facilities, and more...
Conference Highlights are:
Target Audience:
-
Eminent Scientific Professionals in Pharma and Chemistry
-
Faculty (Professors, Associate Professors, Asst. Professors)
-
Pharma and Chemistry Colleges & Training Institutes
-
Pharmaceutical and Chemistry Associations and Societies
-
Pharmaceutical Business Entrepreneurs
-
Manufacturing Pharmaceutical products Companies
-
Manufacturing Medical Devices Companies
-
Pharma and Chemistry Students
-
Ph.D. Scholars, Graduates, and Post Graduates
-
Directors, CEO’s of Organizations
-
Association, Association presidents and professionals
-
Noble laureates in Health Care and Medicine
-
Bio instruments Professionals
-
Bio-informatics Professionals
-
CRO and DATA management Companies
-
Data Management Companies
Conference Series LLC Ltd organizes 800+ Conferences every year across Europe, USA & Asia with support from 1000 more scientific societies and publishes 400+ Open access journals which contain over 50000 eminent personalities, reputed scientists as editorial board members.
For abstract submission please click
We are eagerly waiting to meet you at Amsterdam, Netherlands for the 4th Pharmaceutical Chemistry Conference.
Track 1: Pharmaceutical Chemistry Novel Aspects
Pharmaceutical chemistry is a branch at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various biological specialties, where they participate in the design of drugs, chemical synthesis and development for the market, or bioactive molecules (drugs). In particular, pharmaceutical chemistry in its most common guideline focusing on small molecules / organic entities encompasses synthetic organic chemistry and aspects of natural product chemistry and computational chemistry in close combination with chemical biology, enzymology, and structural biology, together with the goal of discovery and drug development of new different therapeutic agents. Speaking in practical terms, it involves chemical aspects of identification and, subsequently, a systematic and exhaustive synthetic alteration of new chemical entities to make them appropriate for therapeutic use. It includes synthetic and computational aspects of the study of drugs and agents existing in the development in relation to their biological activities (activities and biological properties), that is, the understanding of their structure-activity relationships (SAR). Pharmaceutical chemistry focuses on the quality aspects of medicines and aims to ensure the adequacy of the purposes of the medicines.
-
Physicochemical and biological factors that contribute to drug action
-
Medicinal Radio compounds or Radiopharmaceuticals
-
Global Pharmaceutical Policy
-
Impurities and Impurity Profile Significance in API
-
Design of Safer Chemicals and Products
-
Process Chemistry Considerations
-
Structure-activity relationships of drug Moiety
-
Synthetic chemistry including combinatorial methods
-
In vivo and in vitro biotransformations of drugs
-
Modeling and designing of small compounds
Track 2: Computational Chemistry and Chemical Biology
Computational chemists' daily work effects our accepting of the way the world works, helps manufacturers design more original and efficient procedures, characterizes new compounds and materials, and helps other researchers extract useful knowledge from mountains of data. Computational chemistry is also used to study the important properties of atoms, molecules, and chemical reactions, using quantum mechanics and thermodynamics.
Chemical Biology research uses the tools of chemistry and synthesis to understand biology and disease pathways at the molecular level. Progressive biological chemistry interests include diverse topics such as nucleic acids, DNA repair, bioconjugate chemistry, electron transport, peptides and peptidomimetics, glycoscience, biochemical energy, vitamins, cofactors and coenzymes, biomolecule structure and function, drug activity, imaging, and biological catalysis. Biophysical Chemistry signifies the union of chemistry, physics, and biology using a variety of experimental and theoretical approaches to understanding the structure and function of biological systems.
-
Discovery of biomolecules through metagenomics
-
Chemical approaches to stem-cell biology
-
Metal complexes in medicine
-
Bioorganic and Bioinorganic Chemistry
-
Synthetic biology
-
Enzymology
-
Genome Analysis
-
Chemical and Molecular dynamics
-
Applications of Chemical Biology in Drug Discovery
Track3: Drug Discovery and Therapy
The National Cancer Institute (NCI) provides preclinical cancer research, which includes the evaluation of preclinical safety and the pharmacokinetics of chemotherapeutic agents and preventive chemo. Many research laboratories also provide genetic toxicology tests for the NCI chemoprevention program, synthesize compounds for the NCI carcinogen deposit, and manufacture materials for clinical trials.
National Cancer Institute (NCI) funded by Tumor Glycome Laboratories. They are the main component of the Al-NIH trans Alliance of Glycobiologists for cancer screening and cancer risk, which is looking for glycan-based biomarkers for breast, ovarian, lung, prostate and pancreatic cancer and melanoma.
-
Hit to lead
-
Lead identification and optimization
-
Chemical biology approach to drug discovery
-
Plant-derived drugs in drug discovery
-
Computer-aided drug models in drug discovery
-
Drug transporters in drug discovery
-
A novel paradigm of drug discovery
-
Clinical Drug Development
Track 4: Molecular Stereochemistry
Stereochemistry is the study of the static and dynamic aspects of the three-dimensional shapes of molecules. It has provided a basis for understanding the organic structure and reactivity. In a constant time, the stereochemistry constitutes an associated degree in itself and a fascinating field of analysis in its claim. Several chemists realize this fascinating study space due simply to the aesthetic beauty related to the use of chemical structures, and also the intriguing ability to combine the fields of geometry, topology, and chemistry in the study of three-dimensional shapes. Additionally, there is a square measure of the sensitive ramifications of the stereochemistry. Nature is intrinsically chiral as a result of the building blocks of life (amino acids, nucleotides and sugars) that are chiral squares and that look natural in enantiomerically pure forms. Therefore, any substance created by man to move with or modify the square nature measure interacts with a chiral environment. This is often a very important issue for bio-organic chemists and a sensible problem for pharmaceutical chemists. The Food and Drug Administration (FDA) currently requires that medicine is created in enantiomerically pure forms, or that rigorous tests be performed to confirm that each enantiomer measures in square form.
-
Atropisomerism
-
Cis-trans isomerism
-
Conformational isomerism
-
Diastereomers
-
Enantiomers
Track 5: Pharmacodynamics and Pharmacokinetics
Pharmacodynamics and pharmacokinetics are essentially co-related to each other. Pharmacodynamics is the result that drugs must on the body while pharmacokinetics is the learning of the way in which drugs move through the body during absorption, distribution, metabolism, and evacuation of the body. Clinical pharmacokinetics is the request of pharmacokinetic values to the harmless and real therapeutic administration of drugs in a distinct enduring.
-
Absorption
-
Distribution
-
Biotransformation
-
Excretion
-
General and molecular aspects
-
Drug action
-
Agonistic and antagonistic drug action
Track 6: Genesis of Novel Drugs
The design of computer-aided drugs is the main elementary objective to predict whether a given molecule can or cannot join a target or not. Studying drug design or biomarkers in the style of drugs measures the confirmation of the limited molecule and modeling the conformational changes at intervals, the biological goal that can occur once the bound molecule binds to that. Recent advances in the style of laptop-assisted drugs with the use of high-performance computing technology and the provision of tools and molecular design coding system Insilco would possibly optimize the parameters for the calculations of molecular mechanics and also offer an estimate of the electronic properties (electrostatic potential, polarizability, etc.) of the drug molecule that will influence the binding affinity at intervals of the use of ultrasound in healthy chemistry. Qualitative analysis of high-resolution 1H-NMR and methods of qualitative analysis of X-ray absorption in which it could also be usual to provide semi-quantitative predictions of binding affinity.
-
Modeling and designing of small compounds
-
Computer graphics in drug design
-
Biomarkers in Medical Science
-
Use of High-Performance Computing
-
In-Silico Molecular Design Software and Tools
-
Use of X-ray Crystallography and NMR Spectroscopy in Structure Determination
-
Drug design software (CADD)
Track 7: QSAR in Pharmaceutical Chemistry
The quantitative models of the structure-activity relationship (QSAR models) measure the square regression or the classification models used in the chemical and biological sciences and engineering. Like the alternative regression models, the QSAR regression models relate a collection of "predictor" variables (X) with the efficiency of the response variable (Y), while the QSAR classification models relate the predictor variables with a price categorical of the response variable. In QSAR modeling, predictors contain physicochemical properties or theoretical molecular descriptors of chemical substances; the QSAR response variable may be a biological activity of the chemical products. The initial QSAR models summarize a putative relationship between chemical structures and biological activity in a large amount of chemical product data. Second, QSAR models predict the activities of recent chemical products.
-
QSAR methods: Statistical analysis and physicochemical properties
-
Data mining and knowledge discovery
-
Toxic genomics / Molecular mechanisms
-
Transduction mechanisms and hormonal modulation at the cellular level
-
Predicting environmental toxicity
-
Regulatory Use, Parameters, and Validation of QSAR Models
Track 8: Drug Designing Methodologies
The fundamental problem in the design of computational drugs is to accurately estimate the affinity of ligand-receptor binding. Historically, this deficiency combined with complexity, resources and time requirements has hampered the utility of drug design based on structure.
However, Moore's Law, along with recent advances in GPU-driven computing, has made it possible to obtain accurate results within reasonable time frames.
These calculations are performed alchemically using molecular dynamics to adequately sample an adequate thermodynamic path and intermediate states. The energies measured during the alchemical transformation are then used to computationally calculate the ligand-receptor binding affinity by a series of published techniques, including:
-
Thermodynamic integration
-
A weighted histogram analysis method
-
Bennett acceptance ratio
-
Linear interaction energy
Track 9: Advanced Organic and Inorganic Chemistry
Organic reactions square chemical reactions involving organic compounds. The fundamental chemical reaction classifies square-measure addition reactions, elimination reactions, substitution reactions, pericyclic reactions, preparation reactions, chemical reactions and reaction reactions. In organic synthesis, the square measure of organic reactions is used in the development of recent organic molecules. The assembly of many synthetic chemicals such as medicine, plastics, food additives, and materials depends on organic reactions. There is no limit to the number of viable organic reactions and mechanisms. However, the square measure of the given general patterns that can be used describes several common or useful reactions. Each reaction has a stepwise reaction mechanism that explains, however, how it happens, although this elaborate description of the steps is not invariably clear from a list of the reactants alone. Synthetic organic reactions will be organized in many basic genres. Some reactions coincide with more than one class. For example, some substitution reactions follow a path of elimination of the addition.
In general, the step-by-step progression of the reaction mechanisms will be diagrammatic victimization with arrow-pushing techniques during which the square-shaped curved arrows are used to track the movement of electrons as transition materials to intermediate products and products.
-
Catalyst: Acid and Base Catalyzed Reactions
-
Organometallic Reagents and Compounds
-
Electrophilic and Nucleophilic Substitution
-
Molecular Rearrangements
-
Free-Radical Reactions
-
Transition Metal Catalysis
-
Redox reactions
-
Reactions at ligands
-
Characterization of inorganic compounds
-
Synthetic inorganic chemistry
Track 10: Drug Designing Techniques
Drug delivery systems control the rate at which a drug is released and the location in the body where it is released. Some systems can control both.
Historically, clinicians have attempted to direct their interventions to areas of disease or areas at risk of disease. Depending on the medication, the way it is administered and how our body responds, side effects sometimes occur. These side effects can vary greatly from person to person in type and severity. For example, oral medication for seasonal allergies can cause unwanted drowsiness or an upset stomach.
Administering medications locally rather than systemically (affecting the entire body) is a common way of decreasing side effects and drug toxicity while maximizing the impact of treatment. A topical antibacterial ointment (used on the skin) for a localized infection or a cortisone injection in a painful joint may avoid some of the systemic side effects of these medications. There are other ways to achieve specific drug administration, but some medications can only be administered systemically.
Drug delivery systems are technologies designed for targeted administration and/or controlled release of therapeutic agents.
-
Drug Delivery using Nanotechnology
-
Drug carrier
-
Neural drug delivery systems
-
Acoustic targeted drug delivery
-
Thin film drug delivery
-
Other Controlled Drug Delivery Systems
-
Liposomal and Target Delivery System
-
Beaded Delivery Systems
-
Identification and validation of (emerging) drug targets
-
Strategies for drug delivery to the brain
Track 11: Pharmaceutical Biotechnology and Tissue Engineering
The last few years have been a positive period in general for the pharmaceutical and biotechnology industries. More importantly, there has been a renaissance regarding the increase in the number of new drugs approved and in development for the two business segments.
Many of these innovations are driven by new research methods and the growth of new therapeutic options, such as oncological drugs related to the immune system, personalized medicine, stem cells, and biological products. We are also witnessing the development of a greater number of medicines that cure diseases instead of simply prolonging life.
Valuations of pharmaceutical and biotechnology companies in public markets and mergers and acquisitions increased until August / September of this year, in part due to these positive developments. More recently, there has been a decline in public valuations due to negative publicity about drug prices.
-
Molecular Biotechnology: From DNA Sequence to Therapeutic Protein
-
Production and Purification of Recombinant Proteins
-
Immunogenicity of Therapeutic Proteins
-
Economic Considerations in Medical Biotechnology
-
Monoclonal Antibodies in Various Diseases
-
Biomolecules used to develop targeted drug therapies
-
Biomolecules that elicit specific ligand-receptor interactions
-
Oligopeptides and Nucleic acids
-
Design of proteins
Track 12: Analytical Chemistry& Modern Analytical Techniques
Analytical chemistry is the study of the separation, identification, and quantification of the chemical elements of natural and artificial materials. The qualitative analysis provides a sign of the identity of the chemical species within the sample, and the measurement determines the number of safe elements within the substance. The separation techniques of the elements are commonly performed before the analysis.
Analytical strategies can be separated into classical and instrumental. Classical strategies (also called wet chemistry methods) use separations such as precipitation, extraction and distillation and analysis by color, smell or freezing point. The classic measurement is achieved by measuring the weight or volume. Instrumental methods use an apparatus to measure the physical quantities of the analyte, such as light absorption, fluorescence or conductivity. The separation of materials is carried out by means of mistreatment, natural action or fractionation of field flow.
Analytical chemistry also focuses on improvements in experimental style, chemometrics and, therefore, the creation of the latest measurement tools to produce greater chemical information. Analytical chemistry has applications in forensic medicine, bioanalysis, clinical analysis, environmental analysis and materials analysis.
-
Modern Fluorescence Techniques
-
Modern Spectroscopic Techniques
-
Analytical methods like LC-MS, GC-MS, and NMR
-
Advanced Techniques of Mass Spectroscopy and its Applications
-
Advanced Chromatographic Techniques & its Applications
-
Modern Analytical Instruments
Track 13: Research studies in Pharmacology
Pharmacology is the study of how substances interact with living organisms to produce a change in function. It is about the research, the discovery and the characterization of chemical products that show the biological effects and the illumination of the cellular function and of the organism in relation to these chemical products. If the substances have medicinal properties, they are considered pharmaceutical products. Pharmacology covers the mechanisms of drug action, drug composition and properties, interactions, toxicology, therapies, medical applications, and antipathogenic capabilities.
These are research studies that evaluate how well new medical approaches work in people. Each study answers scientific questions and tries to find better ways to prevent, detect, diagnose or treat a disease. Clinical trials can also compare a new treatment with a treatment that is already available.
The global pharmacy automation market for inpatients and outpatients was valued at $ 2.4 billion in 2011 and should reach $ 2.6 billion in 2012. The total market value is expected to reach almost $ 3.9 billion in 2017 after to increase to a compound annual growth rate (CAGR) of five years 8%.
-
Pharmacological Studies of Herbal Drugs
-
Pharmacological Studies of Medicinal Drugs
-
Electrophysiological and Pharmacological Studies
-
Valid Models for Pharmacological Studies
-
Toxicology
-
Pharmacological Classification of Drugs
-
Pharmacological Classification of Drugs
-
Pharmacological Testing and Research
-
Pharmacological Approaches in Various Treatments
-
Therapeutic Drug Monitoring
Track 14: Heterocyclic Chemistry Compounds
The heterocyclic compound or ring structure can be a cyclic compound having atoms of a minimum of two completely different components as members of its ring (s). Heterocyclic chemistry is that branch of chemistry that deals with the synthesis, properties, and applications of these heterocycles. In distinction, the rings of isocyclic compounds consist entirely of constant-part atoms.
Although the heterocyclic compounds could also be inorganic, most contain a minimum of one carbon. While atoms that measure squares neither carbon nor hydrogen is normally known in organic chemistry as heteroatoms, this can typically be compared to the principal structure of all carbons. However, this does not prevent a compound such as a borazine (which has no carbon atoms) from being labeled "heterocyclic". IUPAC recommends the Hantzsch-Widman language to name heterocyclic compounds.
-
Synthesis of Three-membered ring Derivatives and its Activity
-
Synthesis of Four-membered ring Derivatives and its Activity
-
Synthesis of Five-membered ring Derivatives and its Activity
-
Synthesis of Six-membered ring Derivatives and its Activity
-
Synthesis of Some Polycyclic Heterocyclic Compounds & its Activity
-
Microwave-assisted synthesis of Heterocyclic Derivatives & its Activity
-
Synthesis of New Heterocyclic Mannich Bases and Derivatives
-
Anti-Inflammatory Screening and Molecular Modeling of Some Novel Coumarin Derivatives
Track 15: Green Chemistry in Pharma industry
Green chemistry, also called sustainable chemistry, is an area of chemistry and chemical engineering focused on the design of products and processes that minimize the use and generation of hazardous substances. While environmental chemistry focuses on the effects of chemical pollutants in nature, green chemistry focuses on the environmental impact of chemistry, including technological approaches to prevent pollution and reduce the consumption of non-renewable resources. The general objectives of ecological chemistry, that is, the design of molecules, materials, products, and processes more efficient in terms of resources and inherently safer, can be pursued in a wide range of contexts.
-
Green Buildings, Green Architecture, and Green Engineering
-
Green Nanotechnology
-
Green Economy
-
Environmental Chemistry and Pollution Control
-
Global Warming and Bioremediation
-
Biomass and its Conversion
-
Life Cycle Assessment & Environmental Sustainability
-
Green Analytical Techniques
-
Green Catalysis & Biocatalysts
-
Trends in Green Chemistry
Track 16: Technologies in Natural Products
The discovery of drugs that lead to solid and viable main candidates remains a difficult scientific task, that is, the transition from a selection candidate to a drug candidate needs experience and knowledge. The natural product and its derivatives have been recognized for several years as a supply of therapeutic agents and structural diversity. However, in addition to the diversity of its chemical structure and its diversity, the event of recent technologies has revolutionized the detection of natural products in the discovery of new medicines. The application of these technologies compensates for the inherent limitations of the natural product and offers a new possibility to re-establish the natural product as a significant supply for drug discovery. This topic involves an attempt to explain the use of compounds derived from natural resources as candidates for drugs, with a focus on the success of those resources within the method of finding and discovering new and effective drug compounds, an approach commonly referred to as "natural drug discovery of products".
-
Biosynthetic Mechanisms
-
Prostaglandins and Steriods
-
Alkaloids and Terpenes
-
Synthesis of Amino Acids
-
Peptides & Proteins
-
The Primary Structure of DNA
-
The Secondary & Tertiary Structures of DNA
-
RNA and Protein Synthesis
-
Isolation and Structural elucidation of Natural Products
Track 17: Nanotechnology
Nanotechnology deals with the structure ranging from 1nm to 100nm.It is having a wide range of application in medical fields like drug delivery, Cancer treatment, Imaging, Sensing, Blood purification, Tissue engineering, and Medical devices. It is the operation of matter at atomic and molecular scale to create materials with unusually varied and new properties is a rapidly growing area of research with the vast potential to revolutionize our lives and to provide technological solutions to our problems in agriculture, energy, the environment, and medicine. In order to totally realize this potential, we need to be able to regulator the synthesis of nanoparticles, the production of nano-devices and the classification of materials on the Nanoscale and to understand the effects of these things on environment and health.
-
Nanoparticle
-
Nanomedicine
-
Nanotechnology education
-
Nanotechnology in fiction
-
Nanotechnology in water treatment
-
Nano weapons
-
Nanofluidics
Track 18: Pharmaceutical Drug Impurities
The impurities may be closely related to the product that is formed during the synthesis of a bulk drug or maybe a decomposition product formed during the storage of the drug. The International Conference on Harmonization (ICH) has published guidelines on impurities in new substances, products, and residual solvents. According to the ICH guidelines, an impurity must not exceed 0.1% and the total impurity must not exceed 1.0% in the manufacture of each batch of the drug. The impurities present in excess of 0.1% must be identified and quantified by sufficiently selective methods. The process of identification and quantification of impurities is known as the impurity profile. The process of profiling impurities begins with the detection of impurities by thin layer chromatography, high-performance liquid chromatography or gas chromatography. The presence of impurities in the bulk drug can be identified using the impurity reference standard, which includes the products of predictable side reactions or degradation products. If the retention time of both (impurities present in the reference standard of crude drug and impurities) matches, then the impurities present will be easily identified. In the case of incorrect identification, an analytical method is used, either LC / MS or GC / MS. According to the information of MS, the structure of the impurity will be proposed. The important step in the profiling of impurities is the synthesis of the material (standard of impurities) with the proposed structure. The retention and spectral correspondence of the material synthesized with the impurity present in the bulk drug is useful for the development of the analytical method and the validation of the method. It is essential to know the structure of the impurities present in the bulk drug in order to alter the reaction conditions of the drug and reduce the number of impurities to an acceptable level.
-
Biological Evaluation of Some Known and Unknown Impurities
-
Identification and Physicochemical Characteristics of various Process-Related Impurities
-
Synthesis and Characterization of an Impurities In Bulk Drug
-
Various analytical methodologies to measure impurity levels
-
Various ways to control impurities in pharmaceuticals.
-
Chiral and Polymorphic Impurities
-
Genotoxic and Metabolite Impurities
-
Impurities arising from API–excipient interaction during formulation
Track 19: Overview on Pharmaceutical Industry
Traditional and improved approaches are not mutually exclusive and the competitive industrial environment of today requires the manufacture of medicines that continually strives to improve the quality of the product while reducing production costs. There are numerous pharmaceutical industries around the world that distribute and manufacture medicines where there are advantages beyond a typical contract manufacturing organization (CMO) for the pharmaceuticals market. Many leading pharmaceutical manufacturers and developers have a broad and diverse scientific team that brings a great depth of knowledge and experience. The pharmaceutical industries are making a significant difference in their products from the stages of development to commercialization. A detailed analysis of the industrial structure of contract manufacturing, contract research, and contract packaging was carried out. Revenues are broken down by industry type. The sales figures are estimated for the five-year period from 2013 to 2018. Currently, the United States is spending almost $ 250 billion a year on prescription drugs. If the drugs were sold in a competitive market, without patent monopolies imposed by the government, this could achieve savings of up to $ 200 billion a year.
-
Pharmaceutical Processing Equipment
-
Pharmaceutical Machinery: Tableting, Tube Filling, Liquid Filling, Labeling
-
Pharmaceutical Machinery: Cartoning and Packaging
-
Pharmaceutical Machinery: Standards
-
Pharmaceutical Machinery: Technology
-
Pharmaceutical Manufacturing Equipment
-
Laboratory & Quality Control Equipment
-
Pharmaceutical Machinery: Global Market
Track 20: Pharmaceutical Analysis
Analytical methods must be validated to provide reliable data for regulatory presentations. These methods are essential for a number of purposes, including testing for CC release, stability sample testing, testing of reference materials and for providing data to support specifications. It provides comprehensive coverage of the development of methods and validation requirements to progress in a pharmaceutical compound, at each stage of product development.
It is estimated that the global revenues of advanced drug administration systems will be $ 151.3 billion in 2013. In 2018, it is estimated that revenues will reach almost $ 173.8 billion, which shows a compound annual growth rate (CAGR) ) of 2.8%. Europe contributed about 27% of the total drug delivery market in 2010 and was $ 36 billion. BCC expects this market to grow to $ 49 billion in 2016 at a compound annual rate of 5.6%. The global generic sector reached $ 269.8 billion in 2012. This sector is expected to reach $ 300.9 billion in 2013 and $ 518.5 billion in 2018, with a compound annual growth rate (CAGR) of 11.5%.
-
Various Separation Techniques
-
Electroanalytical methods: Potentiometry and voltammetry
-
Spectroscopy: UV, Mass Spectroscopy and other Spectroscopies techniques
-
NMR Spectroscopy
-
Chromatography techniques
-
Radioanalytical chemistry
-
Gravimetric analysis
-
UPLC and HPLC
-
Titrimetry and X-ray Crystallography
-
Mass Spectrometry
Track 21: Pharmaceutical Marketing
Pharmaceutical Chemistry promoting permits a pharmaceutical company or the selected representative to speak with all persons concerned within the promoting and administration of the pharmaceutical product, as well as the physician, the patient and also the pharmacist. A way of implementing a controlled market includes providing tips, to traders, concerning an award related to initial entity-controlled securities that square measure related to a particular project from a minimum of one in all a plurality of entities come. Planning tools guide promoting professionals through steps of acting a scenario health assessment, distinctive opportunities, developing growth strategies, developing growth techniques, and developing measurements. The planning tools square measure personalized to the answers given by promoting professionals and new tools or changed tools will be simply enforced. These tools will be provided during a complete manner, as a part of a network, and as a part of a cooperative atmosphere. The planning tools ideally type a part of a complete promoting investment manager which incorporates market research management resolution, digital quality management, and hooks to existing systems, like client relationship management, finance, producing, and data technology. The promoting management resolution adopts best practices and includes collaboration, project management, campaign management, and analytics, and measure units.
As a result of the pressure in the market and to maintain sales, there is now, in whose words, “an inherent conflict of interest between the legitimate business goals of manufacturers and the social, medical and economic needs of providers and the public to select and use drugs in the most rational way”. This is particularly true where drugs companies are the main source of information as to which products are most effective.
-
Emerging Pharmaceutical Drugs in Market
-
Providing continuous, ongoing medication therapy management (MTM) for patients
-
Strategic Marketing Planning Processes
-
Identifying, resolving, and monitoring drug-related problems
-
Monitoring the efficacy and safety of medicines
-
Redefining cancer drugs for better and faster treatment innovation in Market
-
Marketing Investment Management
-
Implementation and Analyses of Innovations in Pharmacy Education
Track 22: Clinical and Hospital Pharmacy
The clinical pharmacy is a discipline of health sciences in which pharmacists provide patient care that optimizes drug therapy and promotes health and disease prevention. The medication specialist confirms the legality, well-being and adequacy of the remedy arrangement, verifies the patient's medical history before administering the solution (when such records are kept at the pharmacy), ensures that the prescription quantities are accurately distributed , and choose if the pharmacist should be administered to the patient, with the proper guidance, by a medication specialist. The medication specialist may be interested in courses of action to observe the use of medications, for example, testing test activities and plans to analyze medications to verify adverse drug responses. The subatomic profile of tumor guarantees to propel the clinical administration of malignancy, however, the advantages of coordinating subatomic information with conventional clinical factors have not been deliberately examined. Here we reflexively anticipate tolerant survival using different atomic information (physical modification of the duplicate number).
-
Pharmacotoxicology
-
Hospital Pharmacy& Health practice
-
Specialist Roles in Pharmacy
-
Conference Highlights Biopharmaceutics Clinical Drug Development and Therapeutics Clinical Pharmacology Clinical Pharmacy: Activities and Prescriptions.
The development, production, and marketing of medications are the main aim of pharmaceutical chemistry. Additional years of research and development will bring the drug through clinical trials to finally meet the market strategies. The global market for pharmaceutical contract manufacturing, contract research, and contract packaging will grow from $136.4 billion in 2017 to $197.0 billion by 2022 with a compound annual growth rate (CAGR) of 7.6% for the period of 2017-2022. Pharmaceutical chemistry has dramatically improved the lives of patients The global cancer therapeutics market should reach $172.6 billion by 2022 from $121 billion in 2017 at a compound annual growth rate (CAGR) of 7.4%, from 2017 to 2022.
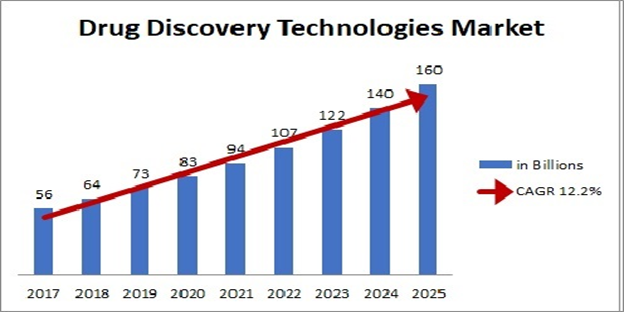
Pharmaceutical Chemistry for Drug Discovery Technologies:
In the sequence of the newest period, the industry's profitability has been decreasing with rising R&D expenditures and time is taken to achieve a cabinet. Healing science is branded as an engaged science that is shaped to cover an extensive variety of fields connected with familiar proof, blend and medication expansion for helpful applications. This science has established from restorative scientists concentrating on calm target or pathway as top importance and suggestion of elaborative science driven events to present day age of high-throughput screening (HTS) libraries that are made by combinatorial science in view of a couple of basic modifications crosswise over vast medication like platforms.
This change was compulsory due to high exhausting down rate in pre-clinical and shading drug improvement thinks about in view of prior systems. While the essential responsibility of therapeutic authorities has not changed basically over a pure stretch of time, the computational plans and chemicals and lookouts at their liberty or evacuation have advanced broadly.
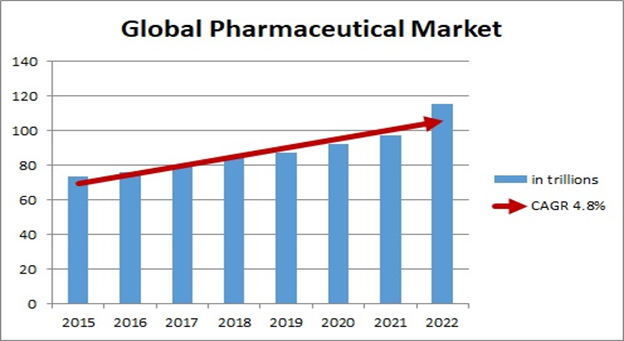
Pharmaceutical Chemistry for Global Pharmaceutical Chemicals: Regional Analysis
North America held the major share of the worldwide pharmaceutical chemicals market in 2016. The growth of the section is mainly accredited to the increase in the request from the customer base in the area.
Europe also detained a giant share of the global pharmaceutical chemicals marketplace in 2016 and is predictable to more grow through the forecast period. The Asia Pacific is predictable to be the wildest rising due to an upturn in the drug trade and customer base. The global marketplace share of North America, Europe, and the Asia Pacific are predicted to increase at a high CAGR during the prediction period, while the global marketplace share of Latin America and the Middle East and Africa is expected to increase, but at a sluggish pace during the prediction period.
Why AMSTERDAM?
Amsterdam is the capital and most populous municipality of the Netherlands. Its status as the capital is mandated by the Constitution of the Netherlands, although it is not the seat of the government, which is The Hague. Amsterdam’s name derives from Amstelredamme, indicative of the city's origin around a dam in the river Amstel. Originating as a small fishing village in the late 12th century, Amsterdam became one of the most important ports in the world during the Dutch Golden Age (17th century), a result of its innovative developments in trade. During that time, the city was the leading Centre for finance and diamonds.
The city is located in the province of North Holland in the west of the country but is not its capital, which is Haarlem. The metropolitan area comprises much of the northern part of the Randstad, one of the larger conurbations in Europe, with a population of approximately 8 million. As the commercial capital of the Netherlands and one of the top financial centers in Europe, Amsterdam is considered an alpha world city by the Globalization and World Cities (GaWC) study group. The city is also the cultural capital of the Netherlands. Many large Dutch institutions have their headquarters there, and seven of the world's 500 largest companies, including Philips, AkzoNobel, TomTom, and ING, are based in the city.
Pharmaceutical Chemistry 2017 Report:
We gratefully thank all our wonderful Keynote and Plenary Speakers, Conference Attendees, Students, Media Partners and Associations for making Pharmaceutical Chemistry 2017 grand success.
The 2nd International Conference on Pharmaceutical Chemistry hosted by the Conference Series LLC Ltd was held during October 02-04, 2017 at TRYP Barcelona Aero Puerto, Barcelona, Spain based on the theme “Recent Trends and Advancements in the fields of Pharma and Chemistry ". Benevolent response and active participation were received from the Organizing Committee Members along with Scientists, Researchers, Students and leaders from the area of Pharmaceutical Chemistry, Chemistry and Analytical science, who made this event a grand success.
Conference Series LLC Ltd expresses its gratitude to the conference Moderator, namely Dr. Matthew D. Lloyd from University of Bath, UK and Dr. Sundaram Singh from Indian Institute of Technology (BHU), India for taking up the responsibility to coordinate during the sessions. We are indebted to your support. Similarly, we also extend our appreciation towards our Poster judges namely, Dr. Catherine Guillou, University of Paris-Saclay, France and Dr. Xianglin Shi, Chemical Process R&D, Biogen Idec, USA.
Conference Series LLC Ltd would like to convey warm gratitude to all the Honorable guests and Keynote Speakers of Pharmaceutical Chemistry 2017:
Catherine Guillou, University of Paris-Saclay, France
Xianglin Shi, Chemical Process R&D, Biogen Idec, USA
Mark McLaughlin, Merck Sharp & Dohme, USA
Gervais Bérubé, Université du Québec à Trois-Rivières, Canada
Larisa Klapshina, Nizhny Novgorod State University, Russia
Fabio Marinelli, University of L’Aquila, Italy
Alexander V. Sirotkin, Constantine the Philosopher University, Slovakia
Conference Series LLC Ltd offers its heartfelt appreciation to organizations such as our esteemed Media Partners, Exhibitors, and other eminent personalities who supported the conference by promoting in various modes online and offline which helped the conference reach every nook and corner of the globe. It also took the privilege to felicitate the Keynote Speakers, Organizing Committee Members, Chairs and Co-chairs who supported this event.
Best Poster Awards Winners:
- Nikolay S. Zimnitskiy, Ural Federal University, Russia
- Mohammed Alqarni, De Montfort University, UK
- Shih-Chieh Yen, Development Center for Biotechnology, Institute of Pharmaceutics, Taiwan
With the grand success of Pharmaceutical Chemistry 2017, Conference Series is proud to announce the “4th International Conference on Pharmaceutical Chemistry" to be held during June27-28, 2019 in Amsterdam, Netherlands.
Let us meet again @ Pharmaceutical Chemistry 2019
For more details visit: https://pharmaceuticalchemistry.annualcongress.com
Conference Highlights
- Pharmaceutical Chemistry Novel Aspects
- Computational Chemistry and Chemical Biology
- Drug Discovery and Therapy
- Molecular Stereochemistry
- Pharmacokinetics and Pharmacodynamics
- Genesis of Novel Drugs
- QSAR in Pharmaceutical Chemistry
- Drug Designing Methodologies
- Advanced Organic and Inorganic Chemistry
- Drug Designing Techniques
- Pharmaceutical Biotechnology and Tissue Engineering
- Analytical Chemistry/Modern Analytical Techniques
- Research studies in Pharmacology
- Heterocyclic Chemistry Compounds
- Green Chemistry in Pharma Industry
- Technologies in Natural Products
- Nanotechnology
- Pharmaceutical Drug Impurities
- Overview on Pharmaceutical Industry
- Pharmaceutical Analysis
- Pharmaceutical Marketing
- Clinical and Hospital Pharmacy
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | June 27-28, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Pharmaceutical Analytical Chemistry
- Modern Chemistry & Applications
- Research & Reviews: Journal of Chemistry
Abstracts will be provided with Digital Object Identifier by